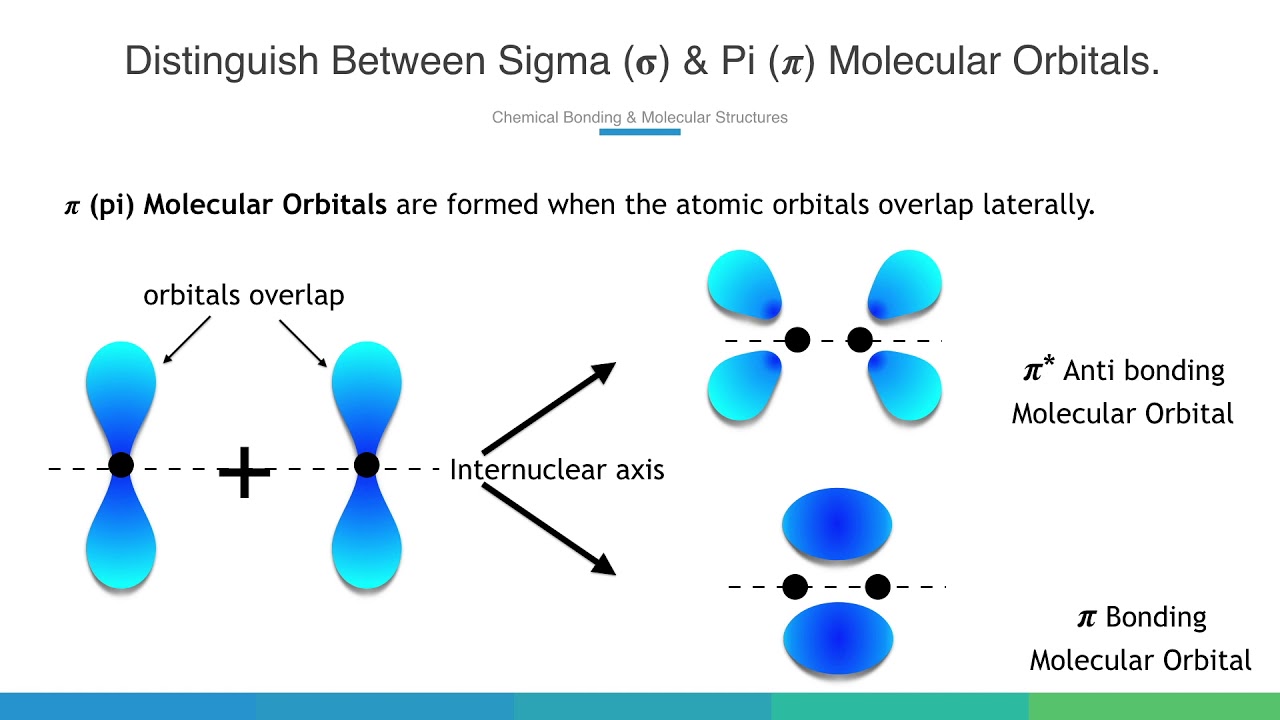
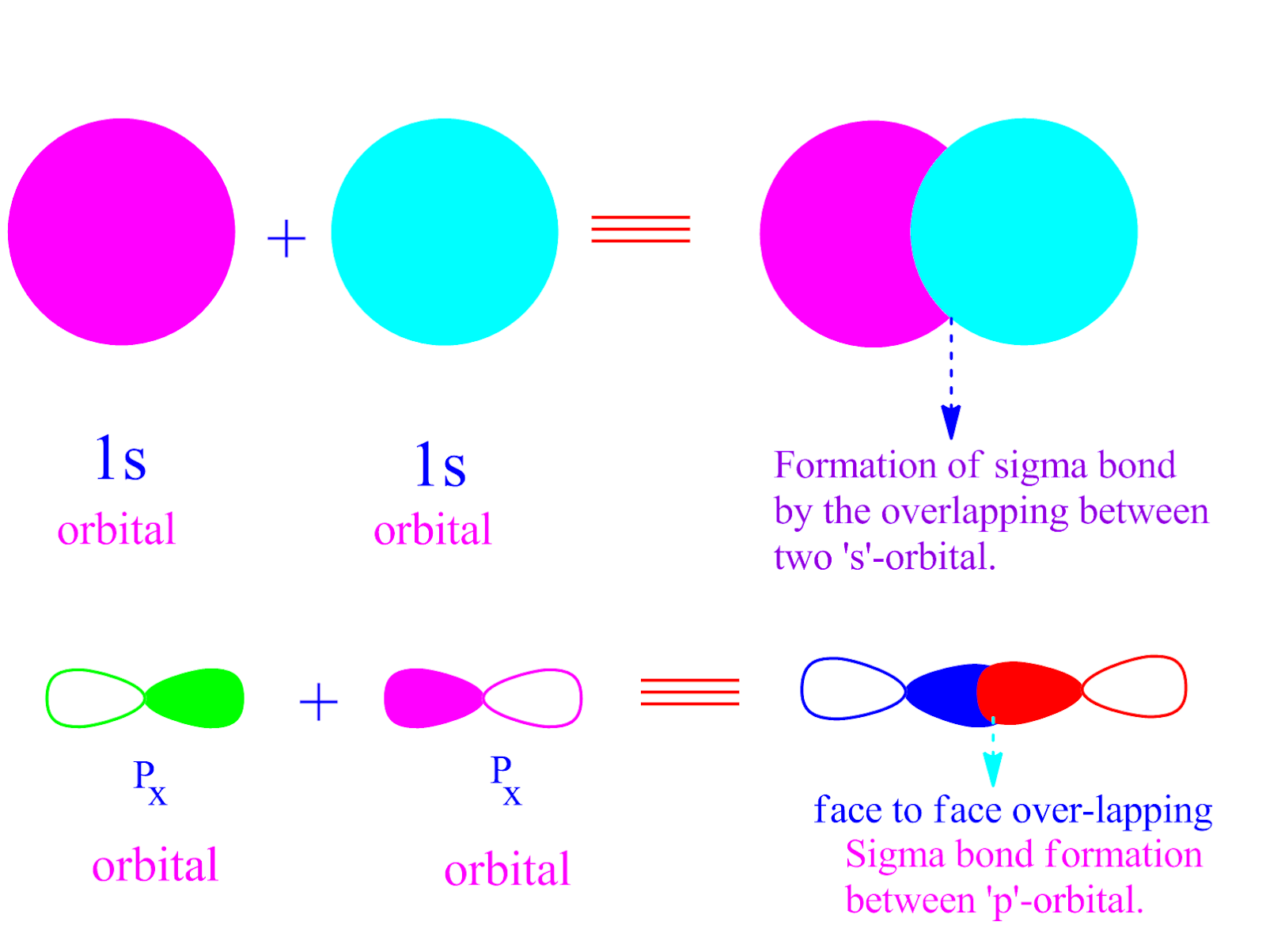
Which Orbitals Form A Pi Bond - To form a pi bond, the orbitals that can overlap are primarily two p orbitals.
To form a pi bond, the orbitals that can overlap are primarily two p orbitals.
To form a pi bond, the orbitals that can overlap are primarily two p orbitals.
Bonding And Antibonding Pi Orbitals Master Organic Chemistry
To form a pi bond, the orbitals that can overlap are primarily two p orbitals.
A π bond is formed by the overlap of
To form a pi bond, the orbitals that can overlap are primarily two p orbitals.
[Solved] Sketch the way orbitals overlap to form the sigma and pi bond
To form a pi bond, the orbitals that can overlap are primarily two p orbitals.
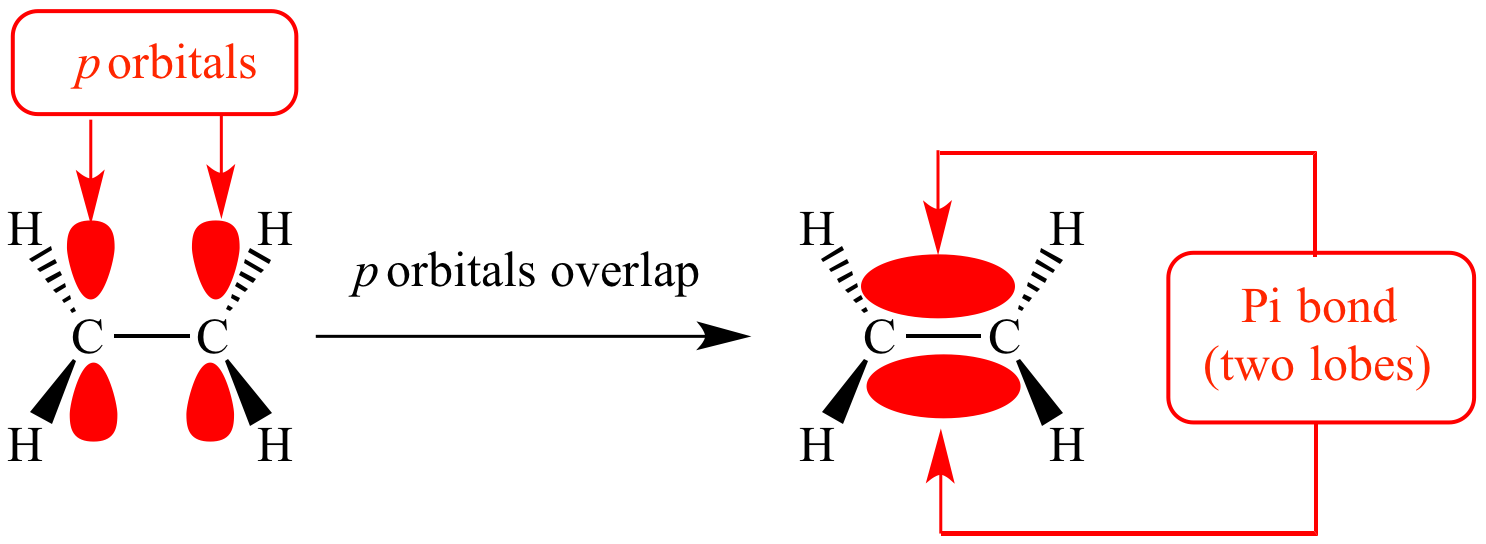
Illustrated Glossary of Organic Chemistry Pi bond
To form a pi bond, the orbitals that can overlap are primarily two p orbitals.
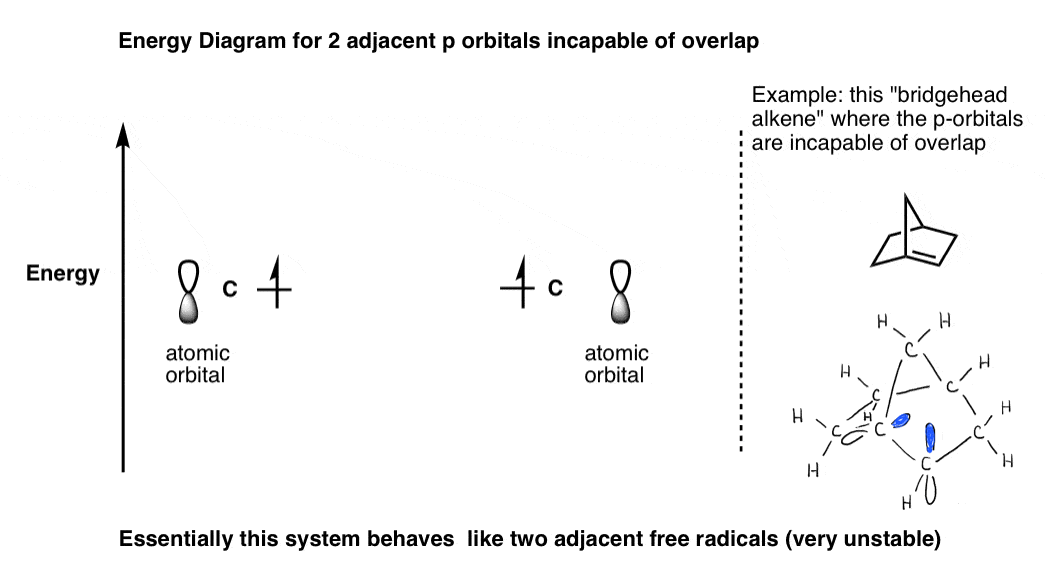
Why can't Sp3 hybrid orbitals can form pi bonds? Socratic
To form a pi bond, the orbitals that can overlap are primarily two p orbitals.
Why are sigma bond more stronger than pi bond ? PG.CHEMEASY
To form a pi bond, the orbitals that can overlap are primarily two p orbitals.
Sigma and Pi Bonds Brilliant Math & Science Wiki
To form a pi bond, the orbitals that can overlap are primarily two p orbitals.
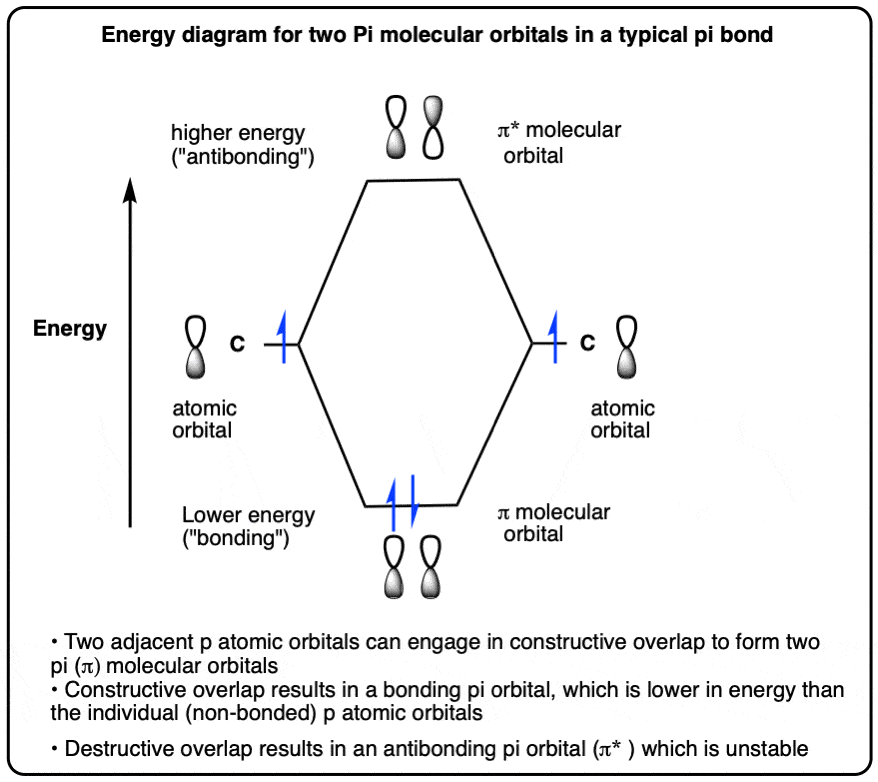
Molecular Orbital Diagram For A Simple Pi Bond Bonding And
To form a pi bond, the orbitals that can overlap are primarily two p orbitals.
How Do You Define a Pi Bond in Chemistry?
To form a pi bond, the orbitals that can overlap are primarily two p orbitals.

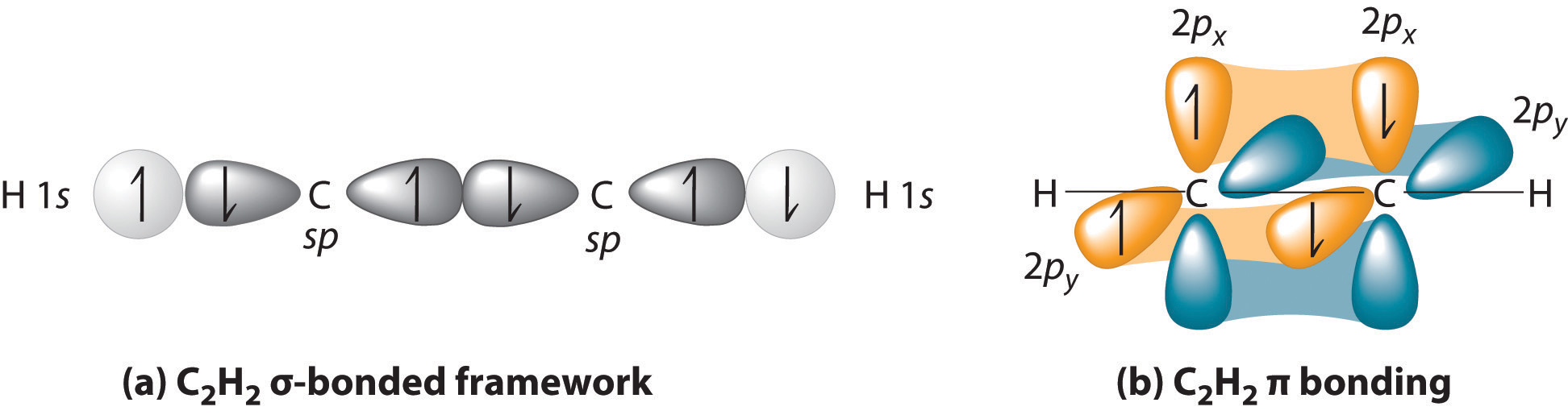

:max_bytes(150000):strip_icc()/1024px-Pi-Bond.svg-d0dece77dabe48c7b13ac7f1ff3d1c4a.jpg)