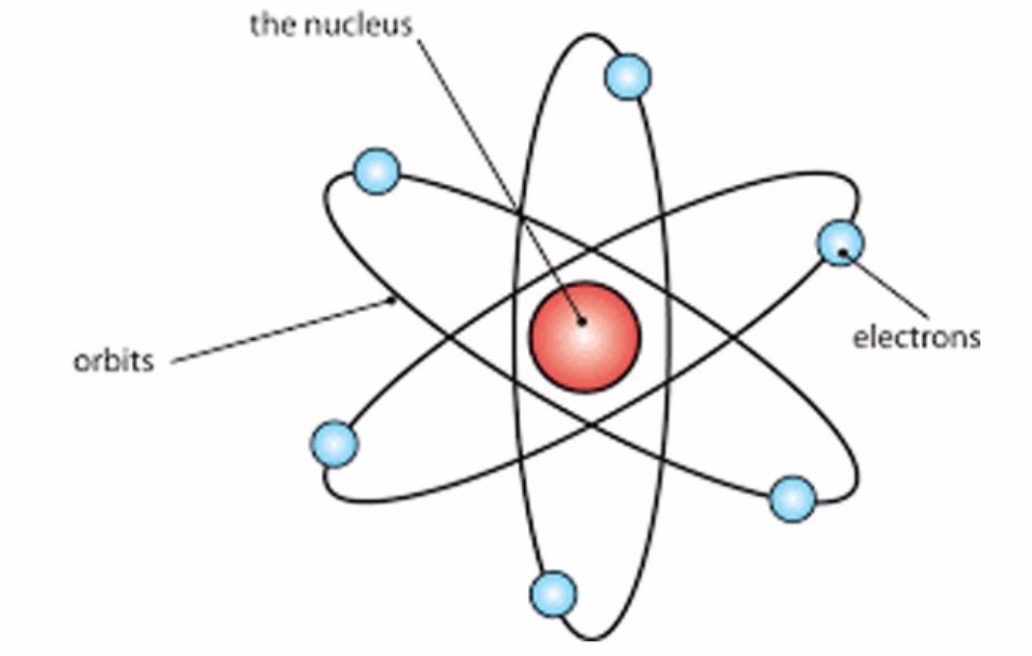
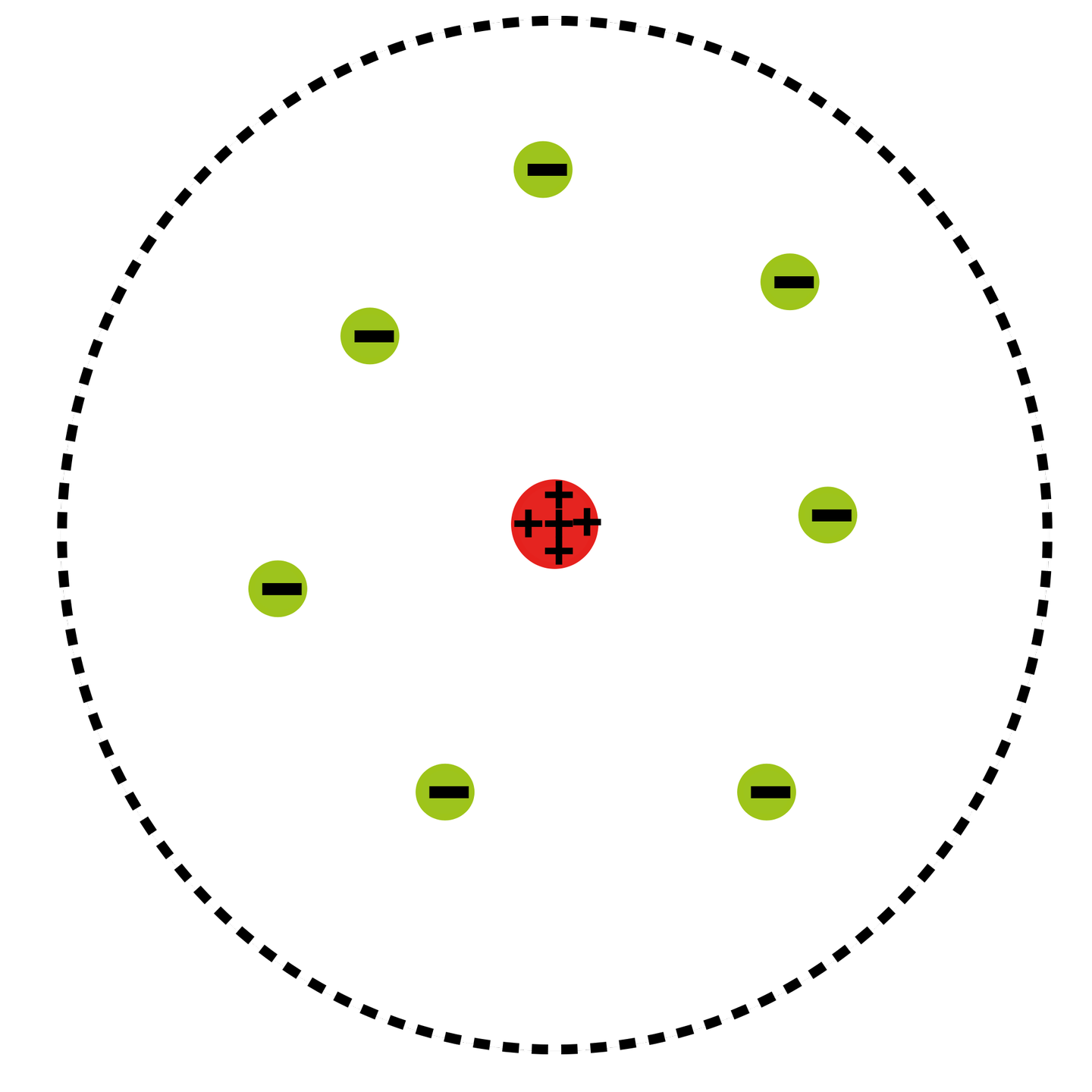
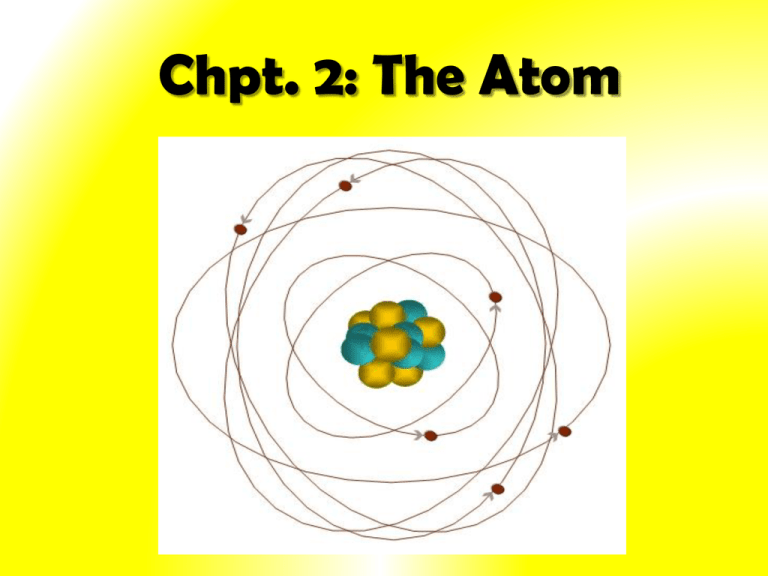
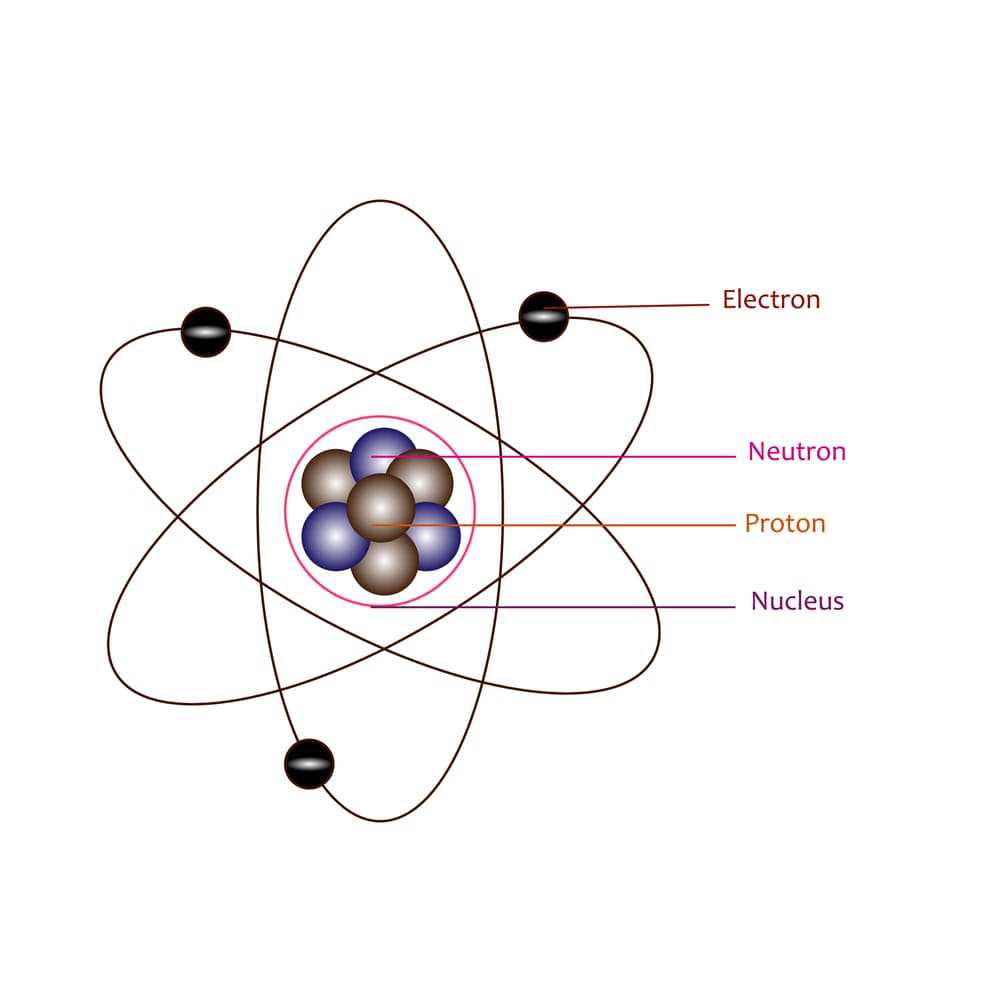
What Was Wrong With Rutherford S Model Of The Atom - The rutherford atomic model was correct in that the atom is mostly empty space. Thus, there must be something drawback in the rutherford’s nuclear model of. Rutherford’s atomic model had the following limitations: Although the rutherford atomic model was based on experimental observations, it failed to. The following are some reasons why rutherford's model was proven wrong:. This atomic model failed to explain.
The following are some reasons why rutherford's model was proven wrong:. This atomic model failed to explain. Rutherford’s atomic model had the following limitations: The rutherford atomic model was correct in that the atom is mostly empty space. Although the rutherford atomic model was based on experimental observations, it failed to. Thus, there must be something drawback in the rutherford’s nuclear model of.
The rutherford atomic model was correct in that the atom is mostly empty space. Thus, there must be something drawback in the rutherford’s nuclear model of. Rutherford’s atomic model had the following limitations: Although the rutherford atomic model was based on experimental observations, it failed to. This atomic model failed to explain. The following are some reasons why rutherford's model was proven wrong:.
Rutherford Atomic Model ChemTalk
The rutherford atomic model was correct in that the atom is mostly empty space. Thus, there must be something drawback in the rutherford’s nuclear model of. Although the rutherford atomic model was based on experimental observations, it failed to. This atomic model failed to explain. The following are some reasons why rutherford's model was proven wrong:.
Rutherford model Definition & Facts Britannica
This atomic model failed to explain. Rutherford’s atomic model had the following limitations: The following are some reasons why rutherford's model was proven wrong:. The rutherford atomic model was correct in that the atom is mostly empty space. Although the rutherford atomic model was based on experimental observations, it failed to.
Rutherford Model of an Atom Class 9, Structure of an atom
The rutherford atomic model was correct in that the atom is mostly empty space. Although the rutherford atomic model was based on experimental observations, it failed to. Rutherford’s atomic model had the following limitations: This atomic model failed to explain. The following are some reasons why rutherford's model was proven wrong:.
Premium Vector Rutherford's model shows that an atom is mostly empty
Thus, there must be something drawback in the rutherford’s nuclear model of. The following are some reasons why rutherford's model was proven wrong:. Although the rutherford atomic model was based on experimental observations, it failed to. This atomic model failed to explain. The rutherford atomic model was correct in that the atom is mostly empty space.
Rutherford Model of Atom Scattering Experiment Structure of Atom
This atomic model failed to explain. Thus, there must be something drawback in the rutherford’s nuclear model of. The rutherford atomic model was correct in that the atom is mostly empty space. The following are some reasons why rutherford's model was proven wrong:. Although the rutherford atomic model was based on experimental observations, it failed to.
SOLUTION Rutherford s model of atom high school 1 2 Studypool
The following are some reasons why rutherford's model was proven wrong:. Thus, there must be something drawback in the rutherford’s nuclear model of. Although the rutherford atomic model was based on experimental observations, it failed to. Rutherford’s atomic model had the following limitations: This atomic model failed to explain.
Rutherford's Model 1898
The rutherford atomic model was correct in that the atom is mostly empty space. Rutherford’s atomic model had the following limitations: Although the rutherford atomic model was based on experimental observations, it failed to. Thus, there must be something drawback in the rutherford’s nuclear model of. This atomic model failed to explain.
Rutherford's Model of the Atom
The following are some reasons why rutherford's model was proven wrong:. This atomic model failed to explain. Thus, there must be something drawback in the rutherford’s nuclear model of. Although the rutherford atomic model was based on experimental observations, it failed to. The rutherford atomic model was correct in that the atom is mostly empty space.
Rutherford Model Images Browse 371 Stock Photos,, 55 OFF
This atomic model failed to explain. The rutherford atomic model was correct in that the atom is mostly empty space. Although the rutherford atomic model was based on experimental observations, it failed to. Rutherford’s atomic model had the following limitations: The following are some reasons why rutherford's model was proven wrong:.
SOLUTION Rutherford s model of atom high school 1 2 Studypool
The rutherford atomic model was correct in that the atom is mostly empty space. Thus, there must be something drawback in the rutherford’s nuclear model of. The following are some reasons why rutherford's model was proven wrong:. Although the rutherford atomic model was based on experimental observations, it failed to. This atomic model failed to explain.
Rutherford’s Atomic Model Had The Following Limitations:
The following are some reasons why rutherford's model was proven wrong:. Although the rutherford atomic model was based on experimental observations, it failed to. This atomic model failed to explain. The rutherford atomic model was correct in that the atom is mostly empty space.