Standard Molar Internal Energy Of Formation - ∗the standard entropy of the h+(aq) ion is defined to be 0. How do you calculate standard molar enthalpy of formation? Since we are adjusting the standard state thermochemical data from table 6.1 the lower. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Definition and explanation of the terms standard state and standard enthalpy of formation, with.
Since we are adjusting the standard state thermochemical data from table 6.1 the lower. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Definition and explanation of the terms standard state and standard enthalpy of formation, with. How do you calculate standard molar enthalpy of formation? ∗the standard entropy of the h+(aq) ion is defined to be 0.
Definition and explanation of the terms standard state and standard enthalpy of formation, with. Since we are adjusting the standard state thermochemical data from table 6.1 the lower. How do you calculate standard molar enthalpy of formation? ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with.
Standard Molar Enthalpy of Formation answer
Definition and explanation of the terms standard state and standard enthalpy of formation, with. Since we are adjusting the standard state thermochemical data from table 6.1 the lower. ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with. How do you calculate standard molar.
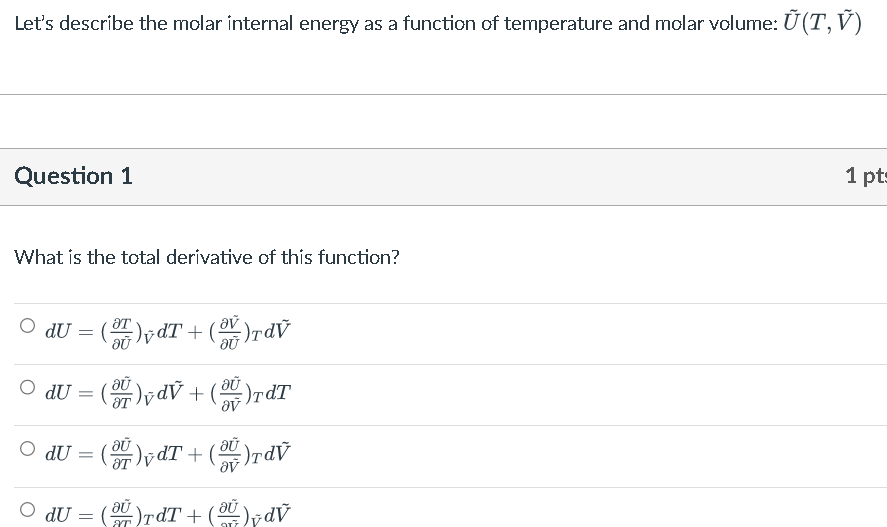
Solved Let's describe the molar internal energy as a
How do you calculate standard molar enthalpy of formation? Definition and explanation of the terms standard state and standard enthalpy of formation, with. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Since we are adjusting the standard state thermochemical data from table 6.1 the lower. ∗the standard entropy of the h+(aq) ion is defined.
Standard Molar Enthalpy of Formation Calculations A Chemistry
Since we are adjusting the standard state thermochemical data from table 6.1 the lower. Definition and explanation of the terms standard state and standard enthalpy of formation, with. How do you calculate standard molar enthalpy of formation? ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy.
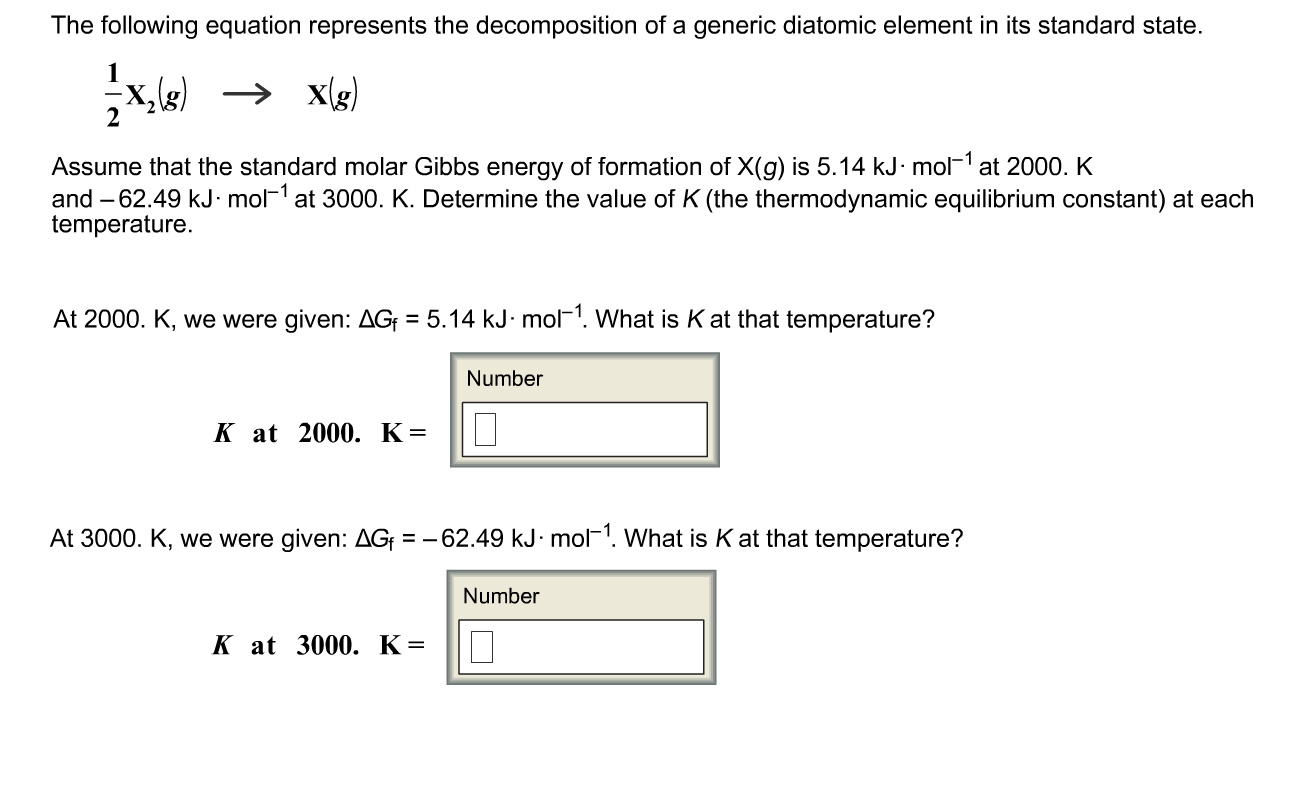
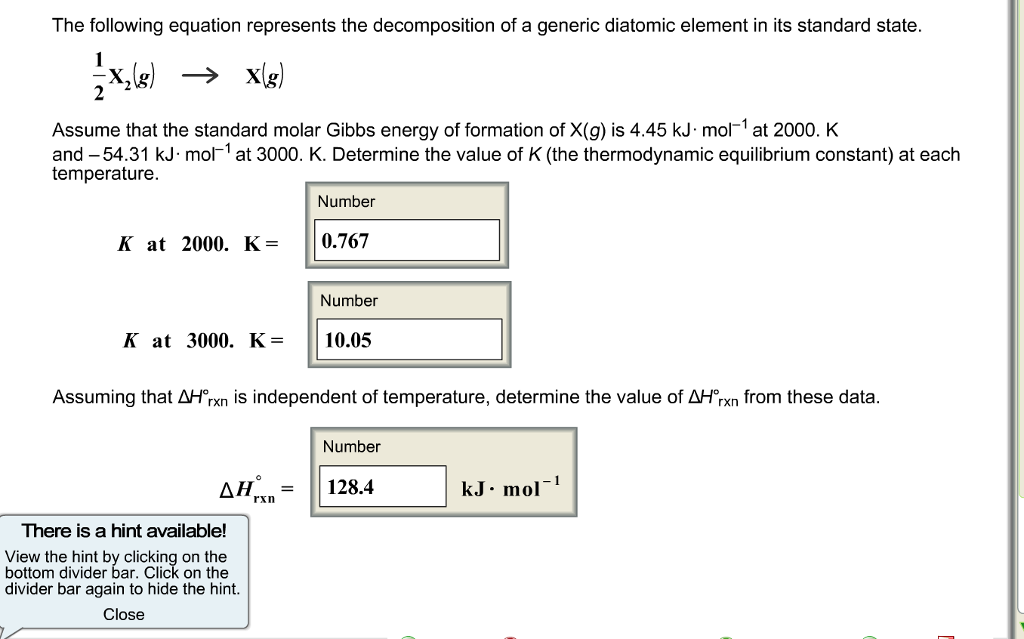
Solved Assume that the standard molar Gibbs energy of
Since we are adjusting the standard state thermochemical data from table 6.1 the lower. How do you calculate standard molar enthalpy of formation? ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Definition and explanation of the terms standard state and standard enthalpy.
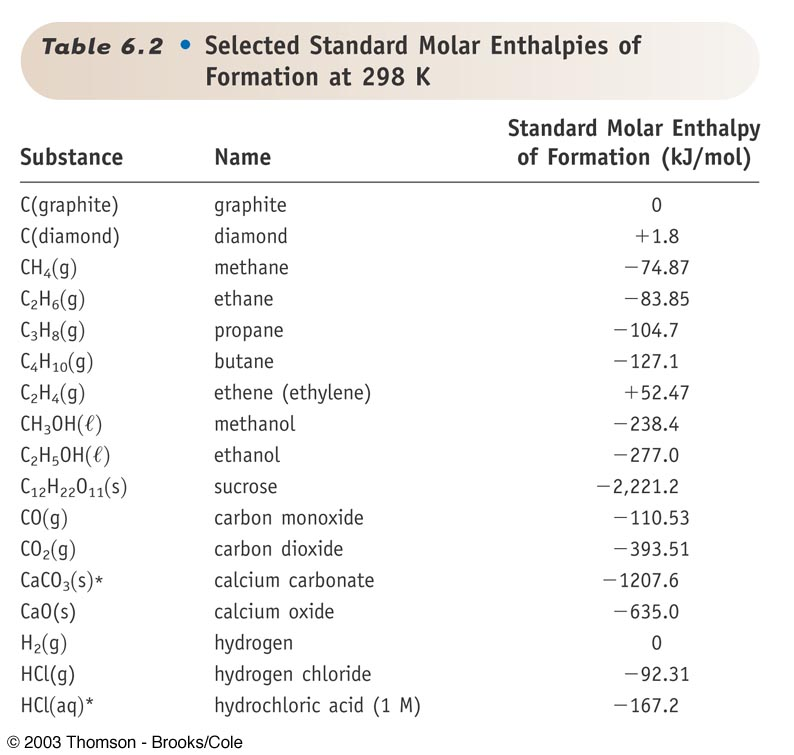
Solved Table 6.2. Selected Standard Molar Enthalpies of
Definition and explanation of the terms standard state and standard enthalpy of formation, with. Definition and explanation of the terms standard state and standard enthalpy of formation, with. ∗the standard entropy of the h+(aq) ion is defined to be 0. How do you calculate standard molar enthalpy of formation? Since we are adjusting the standard state thermochemical data from table.
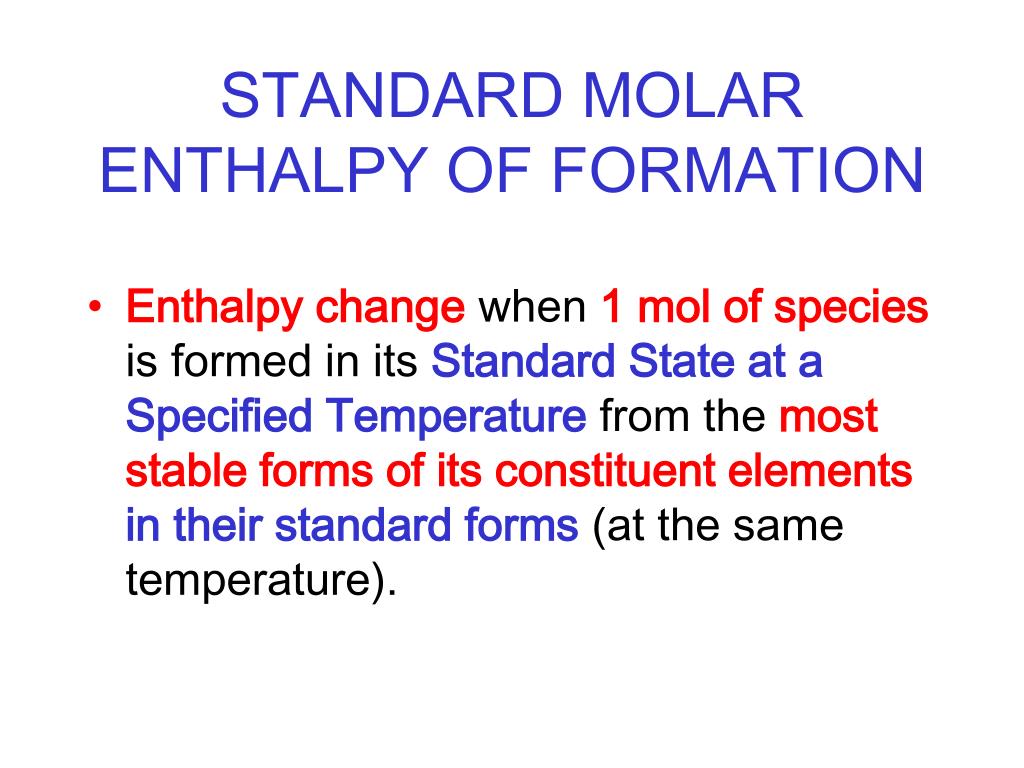
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation
Definition and explanation of the terms standard state and standard enthalpy of formation, with. Definition and explanation of the terms standard state and standard enthalpy of formation, with. How do you calculate standard molar enthalpy of formation? ∗the standard entropy of the h+(aq) ion is defined to be 0. Since we are adjusting the standard state thermochemical data from table.
Using Standard Molar Enthalpies of Formation to Calculate Enthalpy
How do you calculate standard molar enthalpy of formation? ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Since we are adjusting the standard state thermochemical data from table.
Solved Assume that the standard molar Gibbs energy of
How do you calculate standard molar enthalpy of formation? Definition and explanation of the terms standard state and standard enthalpy of formation, with. ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Since we are adjusting the standard state thermochemical data from table.
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation
∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with. How do you calculate standard molar enthalpy of formation? Definition and explanation of the terms standard state and standard enthalpy of formation, with. Since we are adjusting the standard state thermochemical data from table.
Using Standard Molar Enthalpies of Formation
Since we are adjusting the standard state thermochemical data from table 6.1 the lower. ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Definition and explanation of the terms standard state and standard enthalpy of formation, with. How do you calculate standard molar.
Definition And Explanation Of The Terms Standard State And Standard Enthalpy Of Formation, With.
How do you calculate standard molar enthalpy of formation? Since we are adjusting the standard state thermochemical data from table 6.1 the lower. ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with.