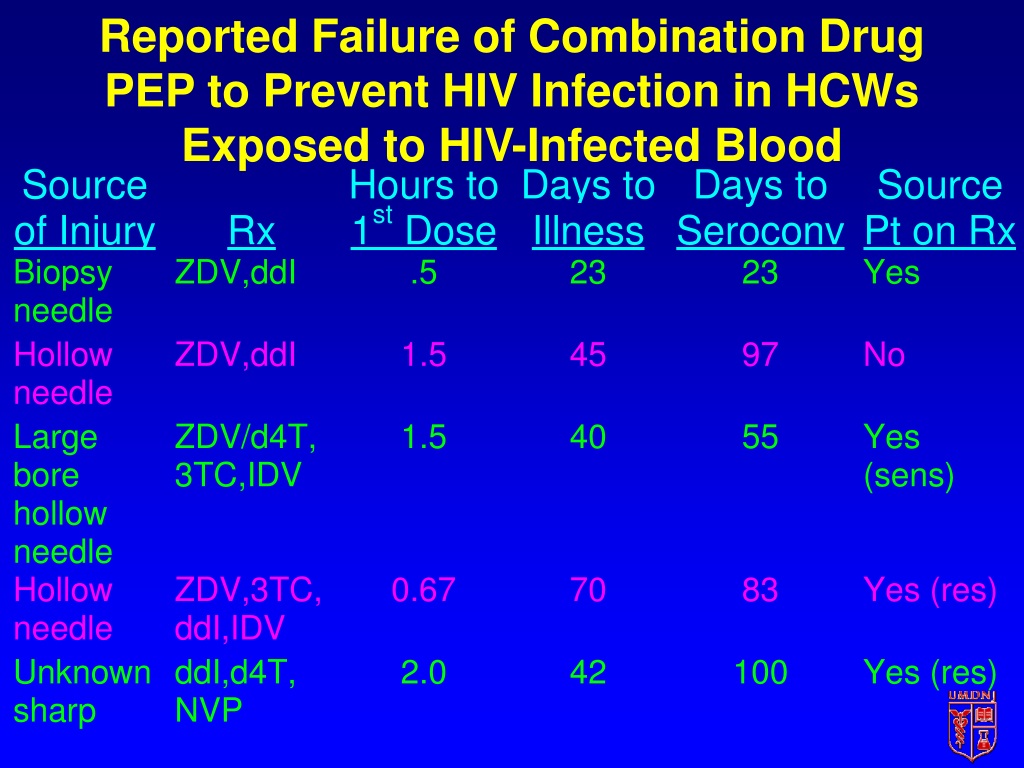
Needlestick Safety And Prevention Act - This landmark legislation updated the office of safety and health administration. The act required that osha amend its. Numerous studies have demonstrated that the use of safer medical devices, such as needleless systems and sharps with engineered sharps injury protections, when they are part of an overall. Osha's bloodborne pathogens standard (29 cfr 1910.1030) as amended pursuant to the needlestick safety and prevention act of 2000, prescribes safeguards to protect workers.
The act required that osha amend its. Osha's bloodborne pathogens standard (29 cfr 1910.1030) as amended pursuant to the needlestick safety and prevention act of 2000, prescribes safeguards to protect workers. Numerous studies have demonstrated that the use of safer medical devices, such as needleless systems and sharps with engineered sharps injury protections, when they are part of an overall. This landmark legislation updated the office of safety and health administration.
Osha's bloodborne pathogens standard (29 cfr 1910.1030) as amended pursuant to the needlestick safety and prevention act of 2000, prescribes safeguards to protect workers. Numerous studies have demonstrated that the use of safer medical devices, such as needleless systems and sharps with engineered sharps injury protections, when they are part of an overall. The act required that osha amend its. This landmark legislation updated the office of safety and health administration.
Solved The Needlestick Safety and Prevention Act is an
Numerous studies have demonstrated that the use of safer medical devices, such as needleless systems and sharps with engineered sharps injury protections, when they are part of an overall. The act required that osha amend its. This landmark legislation updated the office of safety and health administration. Osha's bloodborne pathogens standard (29 cfr 1910.1030) as amended pursuant to the needlestick.
PPT Needlestick Safety and Prevention Act PowerPoint Presentation
Numerous studies have demonstrated that the use of safer medical devices, such as needleless systems and sharps with engineered sharps injury protections, when they are part of an overall. Osha's bloodborne pathogens standard (29 cfr 1910.1030) as amended pursuant to the needlestick safety and prevention act of 2000, prescribes safeguards to protect workers. This landmark legislation updated the office of.
PPT Needlestick Safety and Prevention Act PowerPoint Presentation
This landmark legislation updated the office of safety and health administration. Numerous studies have demonstrated that the use of safer medical devices, such as needleless systems and sharps with engineered sharps injury protections, when they are part of an overall. The act required that osha amend its. Osha's bloodborne pathogens standard (29 cfr 1910.1030) as amended pursuant to the needlestick.
The Needlestick Safety and Prevention Act and SimpleCAP
Osha's bloodborne pathogens standard (29 cfr 1910.1030) as amended pursuant to the needlestick safety and prevention act of 2000, prescribes safeguards to protect workers. Numerous studies have demonstrated that the use of safer medical devices, such as needleless systems and sharps with engineered sharps injury protections, when they are part of an overall. This landmark legislation updated the office of.
PPT Needlestick Safety and Prevention Act PowerPoint Presentation
The act required that osha amend its. This landmark legislation updated the office of safety and health administration. Osha's bloodborne pathogens standard (29 cfr 1910.1030) as amended pursuant to the needlestick safety and prevention act of 2000, prescribes safeguards to protect workers. Numerous studies have demonstrated that the use of safer medical devices, such as needleless systems and sharps with.
Needlestick Safety Prevention Marea Enterprises
The act required that osha amend its. This landmark legislation updated the office of safety and health administration. Osha's bloodborne pathogens standard (29 cfr 1910.1030) as amended pursuant to the needlestick safety and prevention act of 2000, prescribes safeguards to protect workers. Numerous studies have demonstrated that the use of safer medical devices, such as needleless systems and sharps with.
Needlestick Safety And Prevention Act Of 2000
The act required that osha amend its. This landmark legislation updated the office of safety and health administration. Osha's bloodborne pathogens standard (29 cfr 1910.1030) as amended pursuant to the needlestick safety and prevention act of 2000, prescribes safeguards to protect workers. Numerous studies have demonstrated that the use of safer medical devices, such as needleless systems and sharps with.
PPT Needlestick Safety and Prevention Act PowerPoint Presentation
Numerous studies have demonstrated that the use of safer medical devices, such as needleless systems and sharps with engineered sharps injury protections, when they are part of an overall. The act required that osha amend its. This landmark legislation updated the office of safety and health administration. Osha's bloodborne pathogens standard (29 cfr 1910.1030) as amended pursuant to the needlestick.
Fillable Online Needlestick Safety and Prevention Act and the
The act required that osha amend its. Osha's bloodborne pathogens standard (29 cfr 1910.1030) as amended pursuant to the needlestick safety and prevention act of 2000, prescribes safeguards to protect workers. Numerous studies have demonstrated that the use of safer medical devices, such as needleless systems and sharps with engineered sharps injury protections, when they are part of an overall..
Solved Who does OSHA's Needlestick Safety and Prevention Act
Numerous studies have demonstrated that the use of safer medical devices, such as needleless systems and sharps with engineered sharps injury protections, when they are part of an overall. This landmark legislation updated the office of safety and health administration. The act required that osha amend its. Osha's bloodborne pathogens standard (29 cfr 1910.1030) as amended pursuant to the needlestick.
The Act Required That Osha Amend Its.
Numerous studies have demonstrated that the use of safer medical devices, such as needleless systems and sharps with engineered sharps injury protections, when they are part of an overall. Osha's bloodborne pathogens standard (29 cfr 1910.1030) as amended pursuant to the needlestick safety and prevention act of 2000, prescribes safeguards to protect workers. This landmark legislation updated the office of safety and health administration.