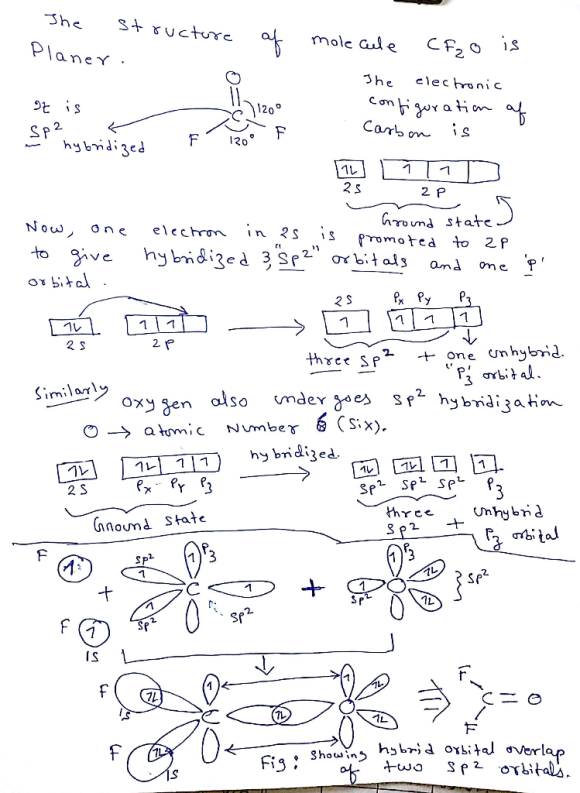
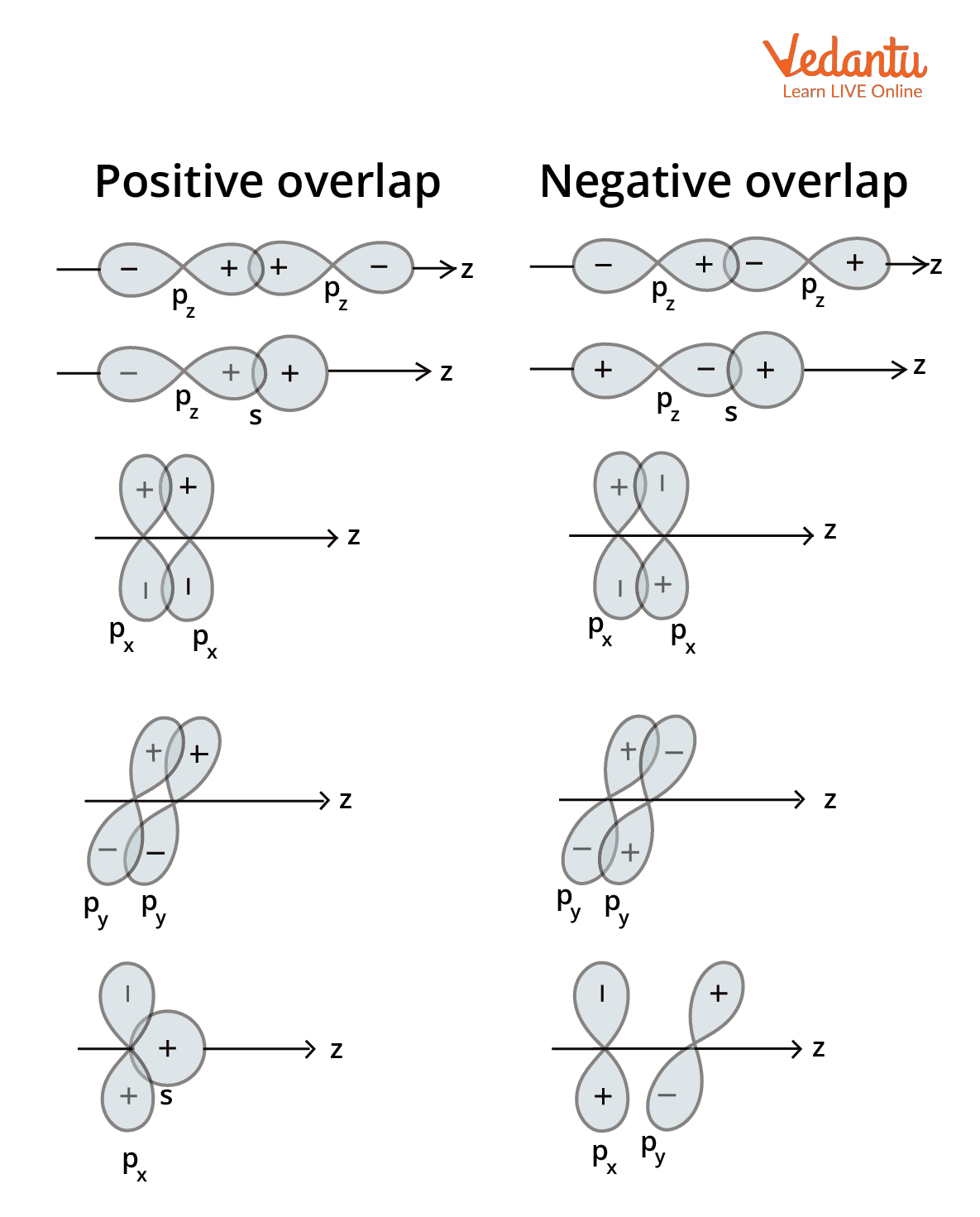
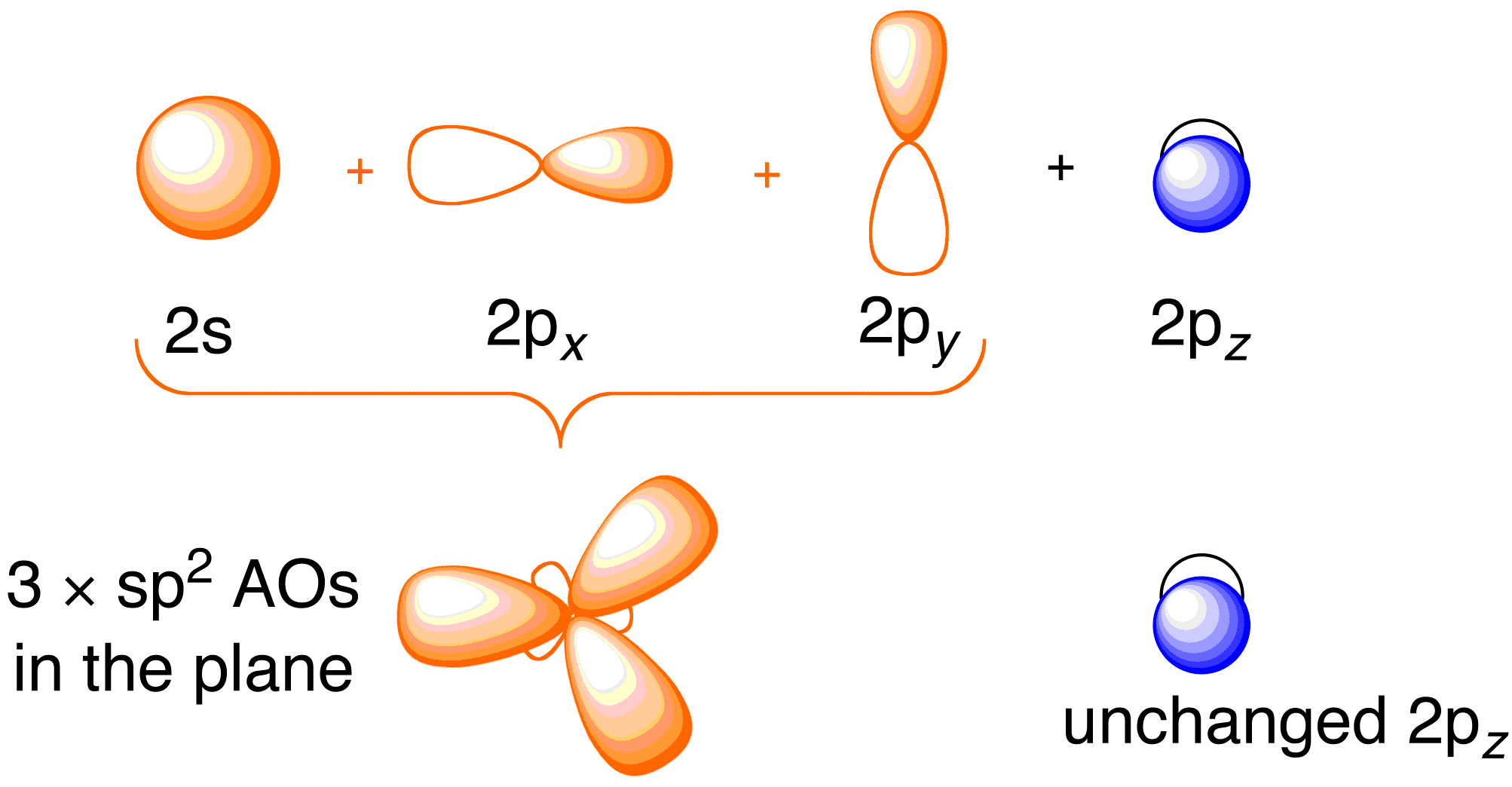Hybrid Orbitals Overlap To Form - The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between. The hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to. Hybrid orbitals have different energies compared to the original atomic orbitals. The overlap of hybrid orbitals or a hybrid orbital and a 1s orbtial from hydrogen creates the.
Hybrid orbitals have different energies compared to the original atomic orbitals. The overlap of hybrid orbitals or a hybrid orbital and a 1s orbtial from hydrogen creates the. The hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to. The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between.
Hybrid orbitals have different energies compared to the original atomic orbitals. The hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to. The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between. The overlap of hybrid orbitals or a hybrid orbital and a 1s orbtial from hydrogen creates the.
Orbital Overlap Diagram
The overlap of hybrid orbitals or a hybrid orbital and a 1s orbtial from hydrogen creates the. The hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to. The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between. Hybrid orbitals have different energies compared.
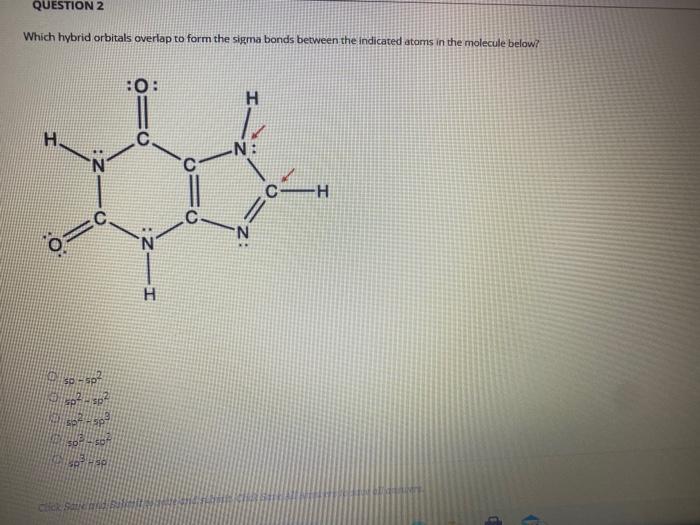
Solved QUESTION 2 Which hybrid orbitals overlap to form the
The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between. The overlap of hybrid orbitals or a hybrid orbital and a 1s orbtial from hydrogen creates the. The hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to. Hybrid orbitals have different energies compared.
Hybrid orbitals
The overlap of hybrid orbitals or a hybrid orbital and a 1s orbtial from hydrogen creates the. Hybrid orbitals have different energies compared to the original atomic orbitals. The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between. The hybrid orbitals overlap to form σ bonds, while the p orbitals on.
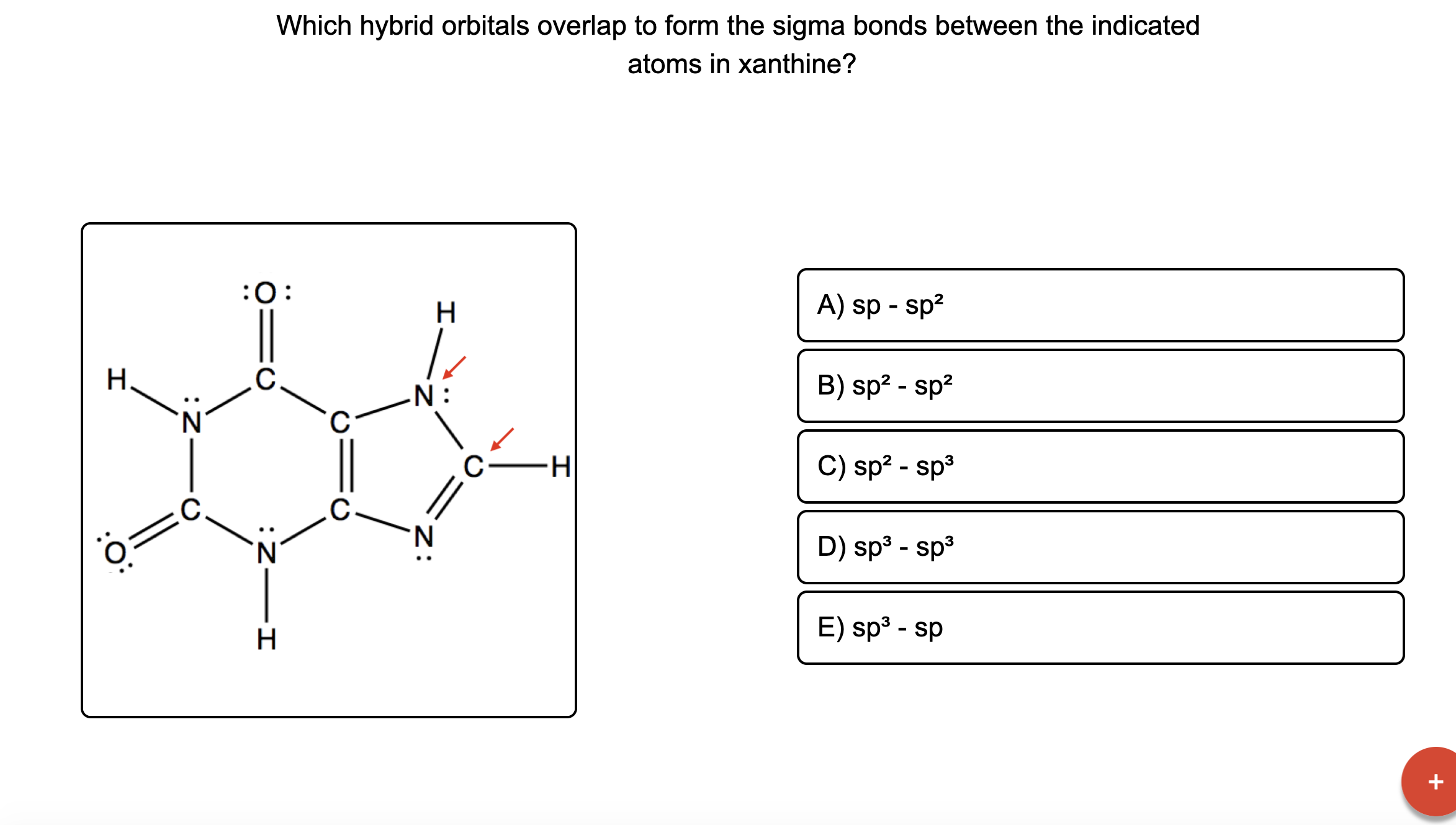
Answered Which hybrid orbitals overlap to form… bartleby
Hybrid orbitals have different energies compared to the original atomic orbitals. The hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to. The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between. The overlap of hybrid orbitals or a hybrid orbital and a 1s.
Hybrid atomic orbitals pianovirt
Hybrid orbitals have different energies compared to the original atomic orbitals. The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between. The overlap of hybrid orbitals or a hybrid orbital and a 1s orbtial from hydrogen creates the. The hybrid orbitals overlap to form σ bonds, while the p orbitals on.
Which hybrid orbitals overlap in the C O bond in CF₂O? Which hybrid
The overlap of hybrid orbitals or a hybrid orbital and a 1s orbtial from hydrogen creates the. The hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to. The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between. Hybrid orbitals have different energies compared.
7.5 Hybrid Atomic Orbitals Chemistry Fundamentals
The overlap of hybrid orbitals or a hybrid orbital and a 1s orbtial from hydrogen creates the. The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between. The hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to. Hybrid orbitals have different energies compared.
7.5 Hybrid Atomic Orbitals Chemistry Fundamentals
Hybrid orbitals have different energies compared to the original atomic orbitals. The hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to. The overlap of hybrid orbitals or a hybrid orbital and a 1s orbtial from hydrogen creates the. The sp hybrid orbitals of the two carbon atoms overlap end to end to.
Orbital Overlap Definition, Detailed Explanation, Properties and FAQs
The hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to. The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between. The overlap of hybrid orbitals or a hybrid orbital and a 1s orbtial from hydrogen creates the. Hybrid orbitals have different energies compared.
Valencebond Orbital Overlap Diagram
The hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to. The overlap of hybrid orbitals or a hybrid orbital and a 1s orbtial from hydrogen creates the. Hybrid orbitals have different energies compared to the original atomic orbitals. The sp hybrid orbitals of the two carbon atoms overlap end to end to.
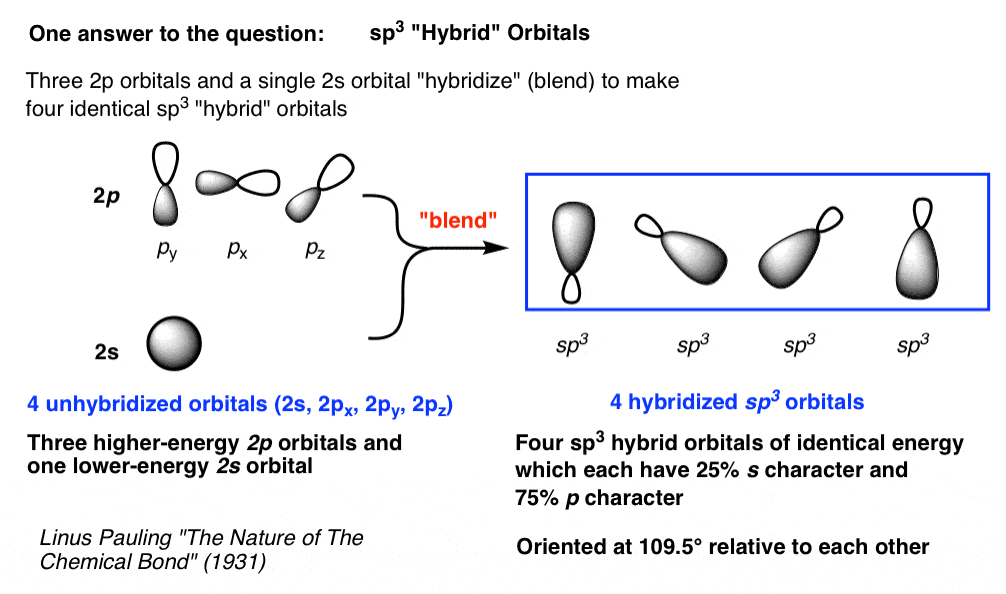
Hybrid Orbitals Have Different Energies Compared To The Original Atomic Orbitals.
The overlap of hybrid orbitals or a hybrid orbital and a 1s orbtial from hydrogen creates the. The hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to. The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between.