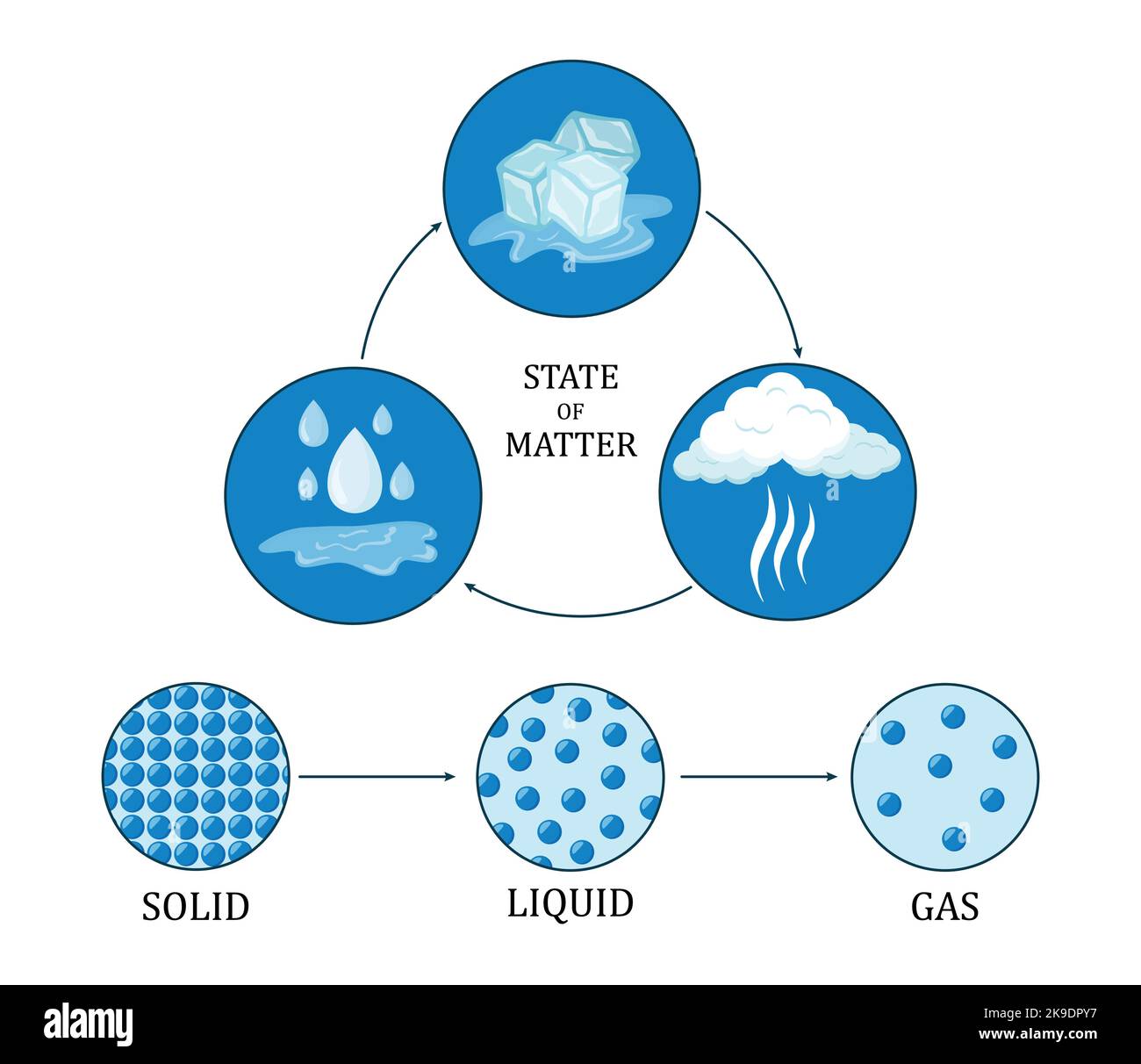
Differentiate Solid Liquid And Gas - Solids are characterized by a fixed shape and volume. Difference between solids, liquids, and gas overview. The particles in solids are tightly packed in an organized arrangement. An object that is rigid, has a low intermolecular space between molecules.
Difference between solids, liquids, and gas overview. Solids are characterized by a fixed shape and volume. An object that is rigid, has a low intermolecular space between molecules. The particles in solids are tightly packed in an organized arrangement.
Solids are characterized by a fixed shape and volume. An object that is rigid, has a low intermolecular space between molecules. The particles in solids are tightly packed in an organized arrangement. Difference between solids, liquids, and gas overview.
SOLUTION Solid liquid gas illustration and example Studypool
The particles in solids are tightly packed in an organized arrangement. Solids are characterized by a fixed shape and volume. Difference between solids, liquids, and gas overview. An object that is rigid, has a low intermolecular space between molecules.
Solid Liquid Gas Project
An object that is rigid, has a low intermolecular space between molecules. Difference between solids, liquids, and gas overview. The particles in solids are tightly packed in an organized arrangement. Solids are characterized by a fixed shape and volume.
What is difference between solid liquid and gas Chemistry Matter
Solids are characterized by a fixed shape and volume. The particles in solids are tightly packed in an organized arrangement. An object that is rigid, has a low intermolecular space between molecules. Difference between solids, liquids, and gas overview.
Different States Of Matter Solid, Liquid, Gas Vector, 60 OFF
Solids are characterized by a fixed shape and volume. An object that is rigid, has a low intermolecular space between molecules. The particles in solids are tightly packed in an organized arrangement. Difference between solids, liquids, and gas overview.
States of Matter As Solid, Liquid and Gas Physical Types Outline
Difference between solids, liquids, and gas overview. The particles in solids are tightly packed in an organized arrangement. Solids are characterized by a fixed shape and volume. An object that is rigid, has a low intermolecular space between molecules.
Solid Liquid Gas Chart
The particles in solids are tightly packed in an organized arrangement. An object that is rigid, has a low intermolecular space between molecules. Solids are characterized by a fixed shape and volume. Difference between solids, liquids, and gas overview.
states of matter, solid, liquid and gas in 2024 Solid liquid gas
The particles in solids are tightly packed in an organized arrangement. Solids are characterized by a fixed shape and volume. An object that is rigid, has a low intermolecular space between molecules. Difference between solids, liquids, and gas overview.
Gas In Liquid Or Solid at William Derr blog
Solids are characterized by a fixed shape and volume. The particles in solids are tightly packed in an organized arrangement. Difference between solids, liquids, and gas overview. An object that is rigid, has a low intermolecular space between molecules.
Properties of Solid, Liquid, Gases A Comparison
An object that is rigid, has a low intermolecular space between molecules. The particles in solids are tightly packed in an organized arrangement. Difference between solids, liquids, and gas overview. Solids are characterized by a fixed shape and volume.
solid liquid and gas posters Made By Teachers
The particles in solids are tightly packed in an organized arrangement. Difference between solids, liquids, and gas overview. An object that is rigid, has a low intermolecular space between molecules. Solids are characterized by a fixed shape and volume.
Solids Are Characterized By A Fixed Shape And Volume.
Difference between solids, liquids, and gas overview. An object that is rigid, has a low intermolecular space between molecules. The particles in solids are tightly packed in an organized arrangement.