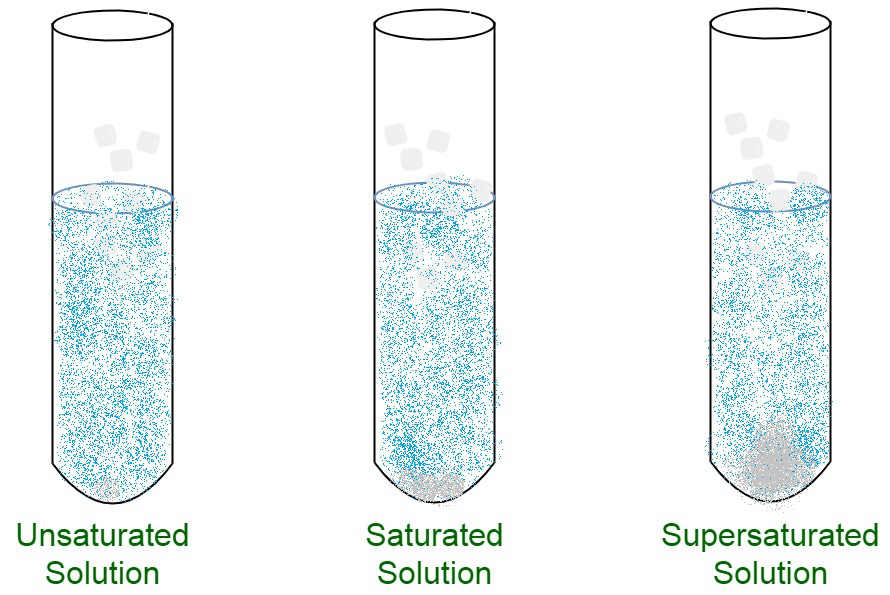
Differentiate Between Saturated And Unsaturated Solutions - (a) differentiate between a saturated and an unsaturated solution. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: An unsaturated solution is a solution. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. How will you test whether a given solution is saturated or not? An unsaturated solution is a solution. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains.
An unsaturated solution is a solution. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. An unsaturated solution is a solution. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. (a) differentiate between a saturated and an unsaturated solution. How will you test whether a given solution is saturated or not?
(a) differentiate between a saturated and an unsaturated solution. How will you test whether a given solution is saturated or not? A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. An unsaturated solution is a solution. An unsaturated solution is a solution. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types:
Saturated and Unsaturated Solutions
The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. (a) differentiate between a saturated and an unsaturated solution. A saturated solution is a solution that contains the maximum amount of solute that is.
Saturated Unsaturated Supersaturated Images
An unsaturated solution is a solution. An unsaturated solution is a solution. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. The main difference between saturated and unsaturated solutions is that.
3. Differentiate between saturated and unsaturated ounds? 4. Write the el..
(a) differentiate between a saturated and an unsaturated solution. An unsaturated solution is a solution. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. How will you test whether a given solution is saturated or not? Depending on the amount of solute dissolved in a given amount of solvent, solutions can.
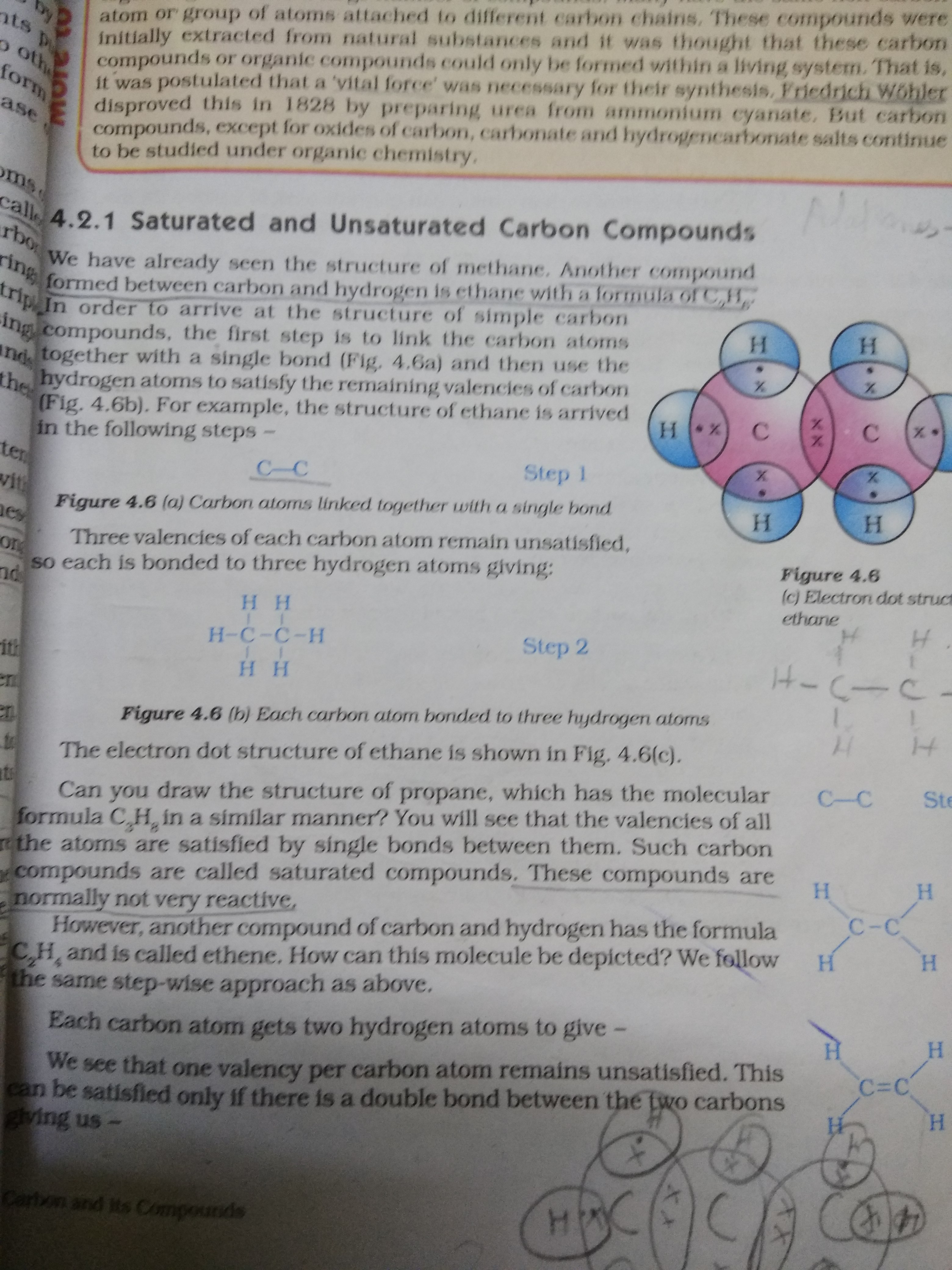
Difference Between Saturated and Unsaturated Compounds Definition
A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: (a) differentiate between a saturated and an unsaturated solution. How will you test whether a given solution is saturated or not? An.
Differentiate between saturated and unsaturated hydrocarbons CBSE
How will you test whether a given solution is saturated or not? The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. An unsaturated solution is a solution. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. Depending on the amount of solute.
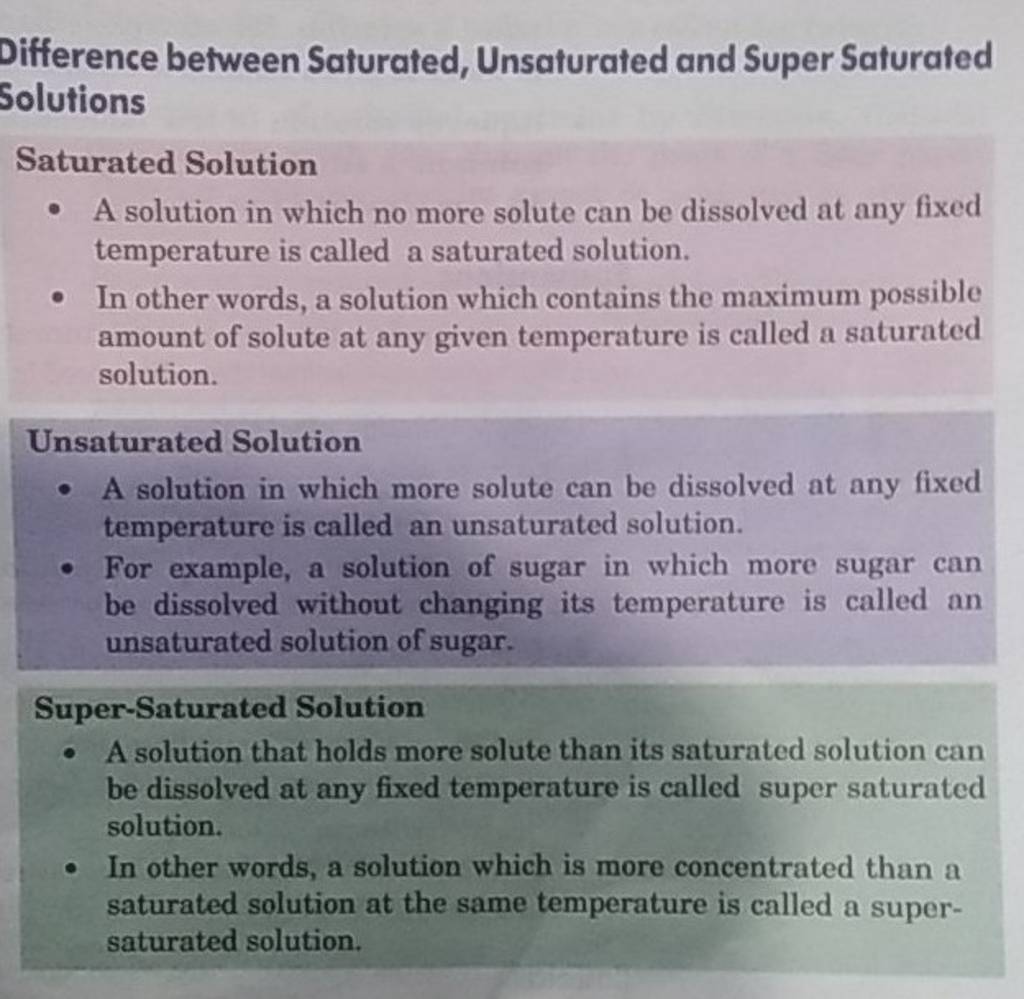
Difference between Saturated, Unsaturated and Super Saturated Solutions S..
A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. An unsaturated solution is a solution. How will you test whether a given solution is saturated or not? Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: A saturated solution is.
Difference Between Saturated and Unsaturated Hydrocarbons Definition
An unsaturated solution is a solution. An unsaturated solution is a solution. How will you test whether a given solution is saturated or not? (a) differentiate between a saturated and an unsaturated solution. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving.
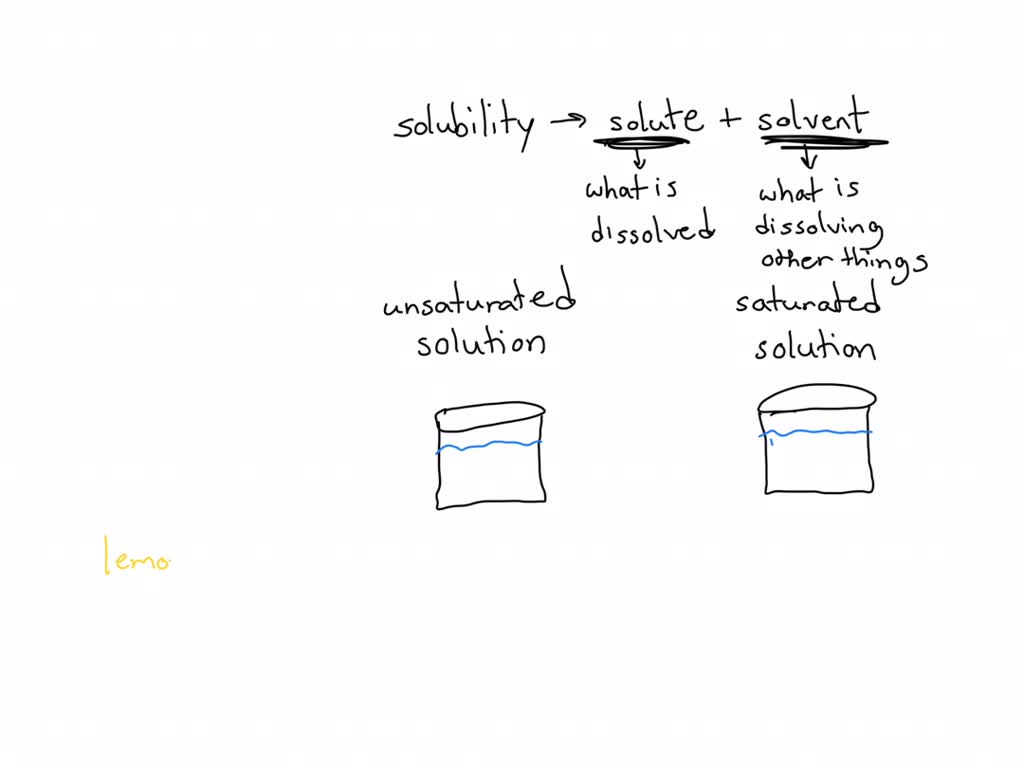
SOLVED Explain the principle of solubility in your own words, and
A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. An unsaturated solution is a solution. The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. (a) differentiate between a saturated and an unsaturated solution. Depending on the amount of solute dissolved in a.
SOLVED Explain the principle of solubility in your own words, and
How will you test whether a given solution is saturated or not? An unsaturated solution is a solution. (a) differentiate between a saturated and an unsaturated solution. A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving. The main difference between saturated and unsaturated solutions is that a saturated solution is a.
Solutions How can one differentiate between saturated unsaturated
How will you test whether a given solution is saturated or not? Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types: The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. An unsaturated solution is a solution. An unsaturated solution is.
A Saturated Solution Is A Solution That Contains The Maximum Amount Of Solute That Is Capable Of Dissolving.
An unsaturated solution is a solution. The main difference between saturated and unsaturated solutions is that a saturated solution is a solution that contains. An unsaturated solution is a solution. Depending on the amount of solute dissolved in a given amount of solvent, solutions can be classified into three types:
(A) Differentiate Between A Saturated And An Unsaturated Solution.
How will you test whether a given solution is saturated or not? A saturated solution is a solution that contains the maximum amount of solute that is capable of dissolving.