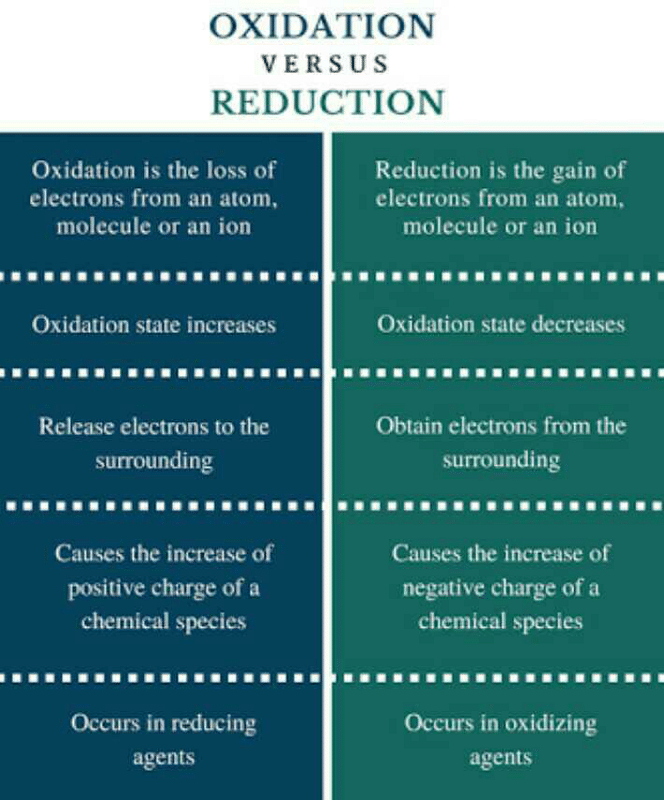
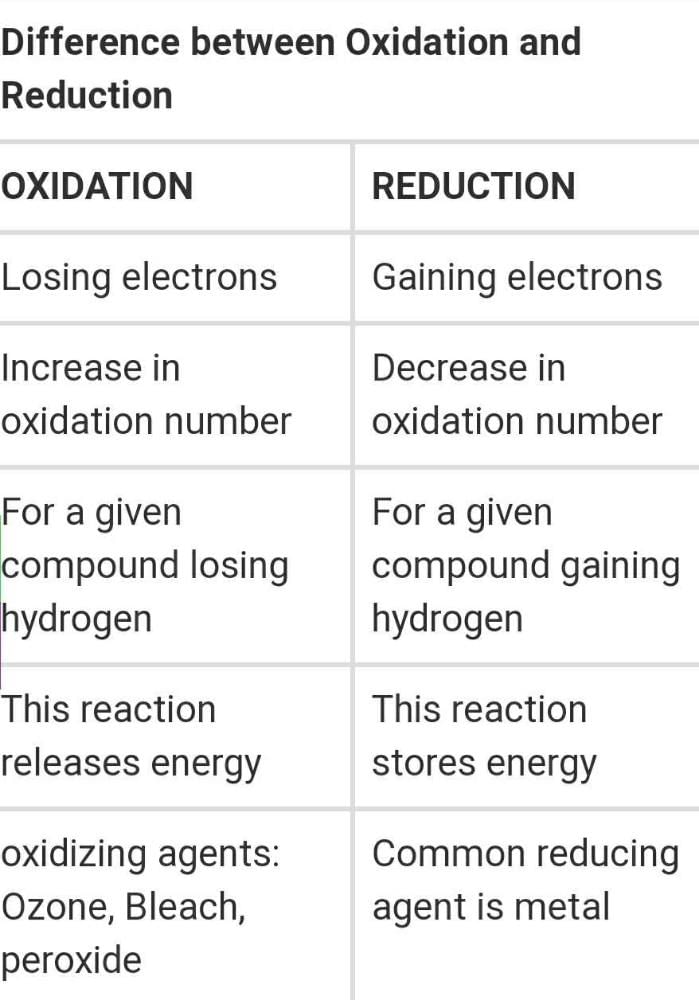
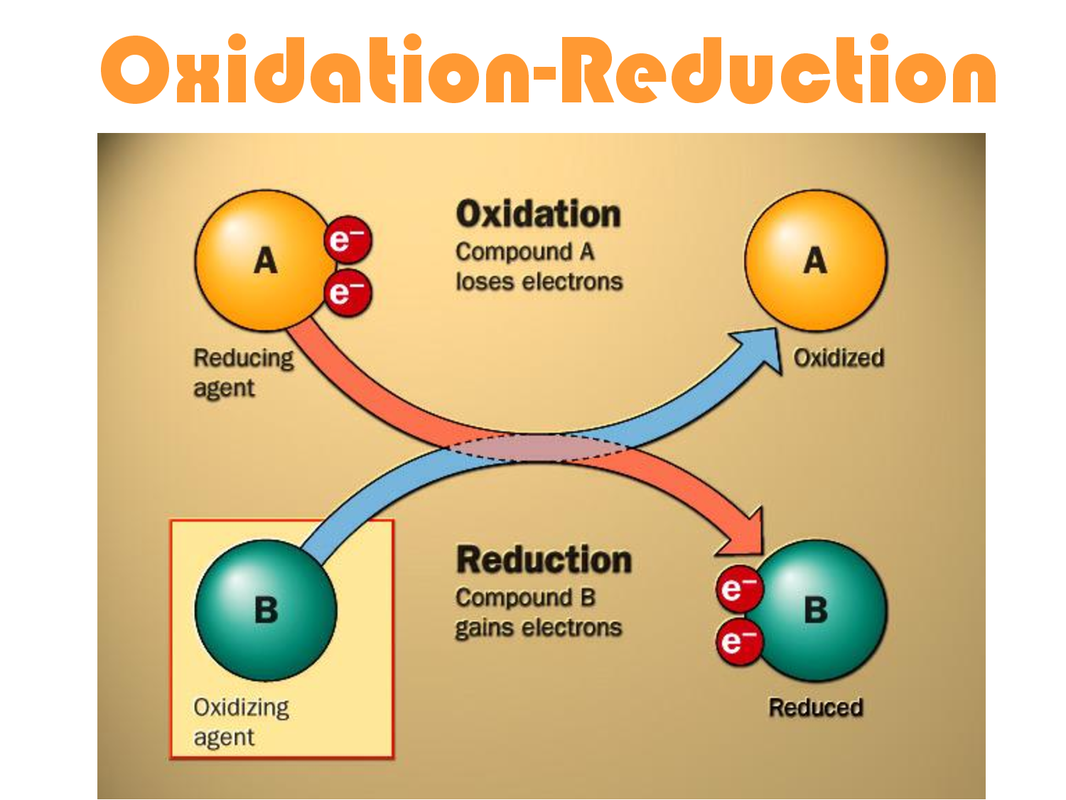
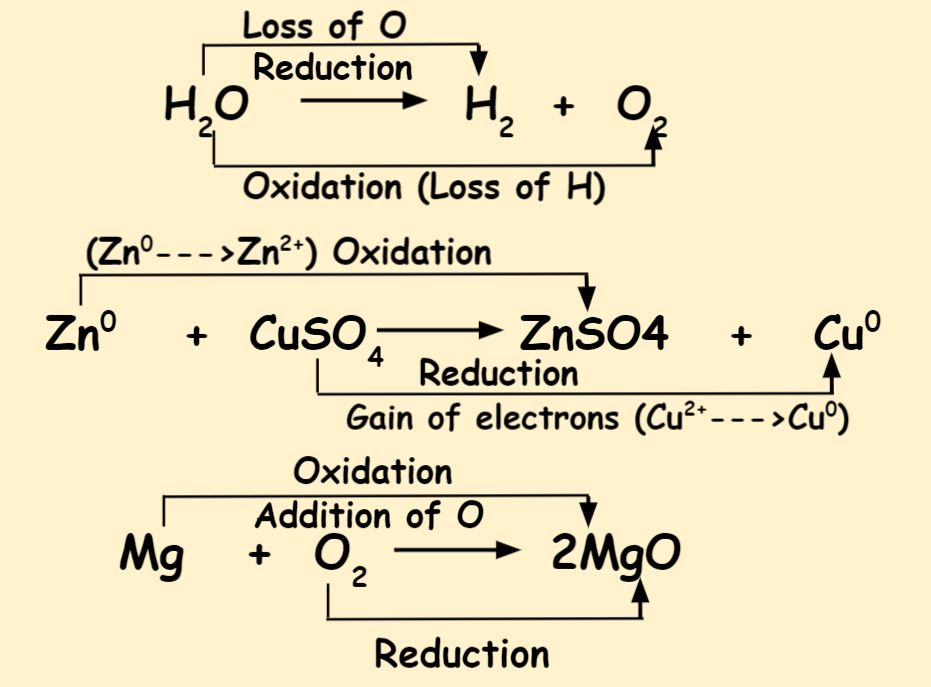
Differentiate Between Oxidation And Reduction - Oxidation and reduction are important chemistry processes that entail electron transport during chemical reactions. Oxidation is the process in which the loss of electrons during a reaction occurs or takes place. Loss of electrons can be done by. The major difference between oxidation and reduction is oxidation is losing of electrons and reduction is gaining of electrons.
The major difference between oxidation and reduction is oxidation is losing of electrons and reduction is gaining of electrons. Oxidation is the process in which the loss of electrons during a reaction occurs or takes place. Loss of electrons can be done by. Oxidation and reduction are important chemistry processes that entail electron transport during chemical reactions.
Loss of electrons can be done by. Oxidation and reduction are important chemistry processes that entail electron transport during chemical reactions. Oxidation is the process in which the loss of electrons during a reaction occurs or takes place. The major difference between oxidation and reduction is oxidation is losing of electrons and reduction is gaining of electrons.
Differentiate between oxidation and reduction? EduRev Class 10 Question
Oxidation is the process in which the loss of electrons during a reaction occurs or takes place. The major difference between oxidation and reduction is oxidation is losing of electrons and reduction is gaining of electrons. Loss of electrons can be done by. Oxidation and reduction are important chemistry processes that entail electron transport during chemical reactions.
Differentiate between oxidation and reduction? EduRev Class 10 Question
Oxidation and reduction are important chemistry processes that entail electron transport during chemical reactions. The major difference between oxidation and reduction is oxidation is losing of electrons and reduction is gaining of electrons. Oxidation is the process in which the loss of electrons during a reaction occurs or takes place. Loss of electrons can be done by.
Oxidation vs Reduction
The major difference between oxidation and reduction is oxidation is losing of electrons and reduction is gaining of electrons. Oxidation and reduction are important chemistry processes that entail electron transport during chemical reactions. Oxidation is the process in which the loss of electrons during a reaction occurs or takes place. Loss of electrons can be done by.
Differentiate between oxidation and reduction reactions Give two
Oxidation and reduction are important chemistry processes that entail electron transport during chemical reactions. The major difference between oxidation and reduction is oxidation is losing of electrons and reduction is gaining of electrons. Loss of electrons can be done by. Oxidation is the process in which the loss of electrons during a reaction occurs or takes place.
Difference Between Oxidation And Vs Reduction Relationship Between
Loss of electrons can be done by. Oxidation and reduction are important chemistry processes that entail electron transport during chemical reactions. Oxidation is the process in which the loss of electrons during a reaction occurs or takes place. The major difference between oxidation and reduction is oxidation is losing of electrons and reduction is gaining of electrons.
TASK 2 OXIDATION AND REDUCTION PRINCIPLES IN BIOCHEMISTRY
The major difference between oxidation and reduction is oxidation is losing of electrons and reduction is gaining of electrons. Oxidation and reduction are important chemistry processes that entail electron transport during chemical reactions. Oxidation is the process in which the loss of electrons during a reaction occurs or takes place. Loss of electrons can be done by.
Difference Between Oxidation and Reduction Compare the Difference
The major difference between oxidation and reduction is oxidation is losing of electrons and reduction is gaining of electrons. Oxidation and reduction are important chemistry processes that entail electron transport during chemical reactions. Oxidation is the process in which the loss of electrons during a reaction occurs or takes place. Loss of electrons can be done by.
10 differences between oxidation and reduction reaction DewWool
The major difference between oxidation and reduction is oxidation is losing of electrons and reduction is gaining of electrons. Loss of electrons can be done by. Oxidation and reduction are important chemistry processes that entail electron transport during chemical reactions. Oxidation is the process in which the loss of electrons during a reaction occurs or takes place.
Difference between oxidation and reduction Teaching chemistry
Oxidation and reduction are important chemistry processes that entail electron transport during chemical reactions. Loss of electrons can be done by. The major difference between oxidation and reduction is oxidation is losing of electrons and reduction is gaining of electrons. Oxidation is the process in which the loss of electrons during a reaction occurs or takes place.
SOLVED Differentiate between oxidation and reduction (2)
Oxidation is the process in which the loss of electrons during a reaction occurs or takes place. Loss of electrons can be done by. The major difference between oxidation and reduction is oxidation is losing of electrons and reduction is gaining of electrons. Oxidation and reduction are important chemistry processes that entail electron transport during chemical reactions.
Loss Of Electrons Can Be Done By.
The major difference between oxidation and reduction is oxidation is losing of electrons and reduction is gaining of electrons. Oxidation and reduction are important chemistry processes that entail electron transport during chemical reactions. Oxidation is the process in which the loss of electrons during a reaction occurs or takes place.